Abstract

Background: Only few studies have validated the use of molecular minimal measurable residual disease evaluation using error-corrected NGS (NGS-MRD). The prognostic relevance of detection of isolated DNMT3A, TET2 or ASXL1 (DTA) mutations post-induction remains controversial. Benchmarking of NGS-MRD with respect to other molecular MRD tools (such as WT1 expression and NPM1 mutation) has yet to be conducted.
Material and methods: All patients included in the ALFA-0702 study who reached cytological complete remission (CR) or CR with incomplete platelets recovery after one induction course and with available material were included (n=189). Standard NGS sequencing of 67 genes was performed at diagnosis. Error-corrected NGS was performed on post-induction bone marrow samples using a custom panel including all mutations identified at diagnosis (n=735 mutations in 51 genes). Median sequencing depth in CR was 24695x after UMI deduplication. A threshold of 0.1% VAF was set as cut-off value for NGS-MRD detection for all targets. WT1 expression data in CR were available in 100 patients with initial WT1 over-expression. NPM1-MRD was evaluated in 67 patients by error-corrected NGS. For this evaluation, one alternative read was considered sufficient to define positivity. Leukemia free survival (LFS) and overall survival (OS) probabilities were evaluated since CR.
Results: Median age at diagnosis was 46 years. ELN2017 risk was favorable in 52 (27%) patients, intermediate in 75 (40%) and unfavorable in 60 (32%). With a median follow-up from CR of 47 months, median LFS and OS was 35 and 41 months respectively. At diagnosis, gene mutations were detected in 181 (95%) patients. The median number of mutations per patient was 4 (range 1-10).
Ninety-one patients had no target detected (NGS-MRD neg) and ninety had at least one target detected (NGS-MRD pos) including 42 patients with detection of DTA mutations only (DTA-MRD). NGS-MRD pos patients were significantly older (p=0.0018) than NGS-MRD neg patients and harbored a higher median number of mutations at diagnosis (4 vs 3, p=0.0021). There were no significant differences in terms of demographics and clinical presentation (age, WBC, number of mutation) between patients with DTA-MRD and non-DTA-MRD patients.
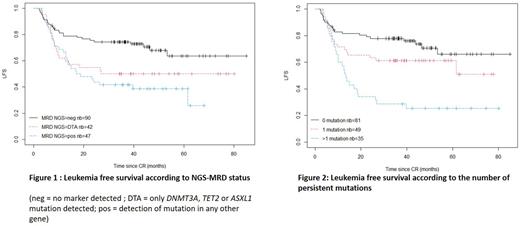
In univariable analyses, NGS-MRD pos was associated with a lower probability of LFS (4-years LFS: 44% vs 68%, p= 6e-04) and OS (4-years OS: 57% vs 81%, p= 9e-04). There was no impact of the initial number of mutations on LFS nor OS. There were no significant differences in LFS and OS between DTA-MRD and non-DTA-MRD patients (figure 1). When adjusting analysis on ELN2017 status, MRD-NGS pos remained associated with shorter LFS (HR 2.15[1.34-3.44] , p=0.001) and OS (HR 2.41[1.38-4.20] p=0.002). As a continuous variable, the number of mutations detected in CR was associated with shorter LFS and OS. Specifically, detection of ≥2 mutations predicted poorer LFS (HR, p=3e-05) and OS (HR, p=4 e-05), compared with detection of only 1 mutation (figure 2).
Sixteen patients were positive for WT1 MRD evaluation, and 84 were negative (WT1 neg). Among the WT1 neg patients 37 were NGS-MRD pos and 47 were NGS-MRD neg. When adjusting for both ELN17 and WT1 expression in CR, there was no significant impact of MRD-NGS positivity on LFS (HR=1.62 [0.85 - 3.09]) nor OS (HR=1.28 [0.59 - 2.74])
In the 67 patients with NPM1 mutation, 41 patients had NPM1 undetectable MRD (including 21 NGS-MRD pos and 20 NGS-MRD neg) and 26 had detectable NPM1-MRD (including 16 NGS-MRD pos and 10 NGS-MRD neg). When adjusting for both ELN status and NPM1-MRD, NGS-MRD pos was associated with a non-significant trend towards lower LFS (HR=2.34[0.96-5.71] p=0.063).
Discussion and conclusions: In the homogeneously treated ALFA-0702 population, MRD-NGS positivity after the first induction course predicts shorter LFS and OS independently of ELN17 risk stratification. Detection of a higher number of mutations in CR is associated with a worse prognosis. Contrary to previous studies accruing older patients treated less intensively persistence of DTA mutations has similar prognostic relevance than non-DTA mutations. Future studies will be necessary to determine the superiority or addivitity of NGS-MRD with respect to available molecular MRD tools.
Disclosures
Hirsch:novartis: Consultancy. Lambert:Astellas: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal